Mechanism of Action:
Piracetam improves membrane stability, allowing the membrane and transmembrane proteins to maintain or recover the three-dimensional structure to exert their function. Piracetam has neuronal and vascular effects. Pharmacokinetics:
Absorption:
Piracetam is rapidly and extensively absorbed following oral administration. In fasted subjects, the peak plasma concentrations are achieved 1 hour after dosing. The absolute bioavailability of piracetam oral formulations is close to 100%. Food does not affect the extent of absorption of piracetam.
Distribution:
Piracetam is not bound to plasma proteins and its volume of distribution is approximately 0.6 l/kg. Piracetam crosses the blood brain barrier as it has been measured in cerebrospinal fluid following intravenous administration. Metabolism:
Piracetam is not known to be metabolised in the human body. This lack of metabolism is supported by the lengthy plasma half-life in anuric patients and the high recovery of parent compound in urine.
Elimination:
The plasma half-life of piracetam in adults is about 5 hours following either intravenous or oral administration.
The apparent total body clearance is 80-90 ml/min. The major route of excretion is via urine, accounting for 80 to 100% of the dose. Piracetam is excreted by glomerular filtration.
Indications:
Studies carried out in the elderly suffering from loss of memory, vertigo, a lack of concentration or of alertness, changes of mood, a deterioration in behaviour and personal negligence, demonstrate an improvement in symptoms.
These symptoms can also provide an early warning of the onset of pathological ageing such as Alzheimer’s Disease, an Alzheimer type of senile dementia, or the dementia produced by multiple cerebral infarcts. Piracetam is advocated in the treatment of sickle-cell vaso-occlusive crises.
Studies have shown some improvement in children with learning difficulties associated with the written word, particularly with textual understanding which cannot be explained by intellectual backwardness, inadequate education or by the family environment. The administration of Piracetam does not replace other measures also well adapted to correct these learning difficulties, such as remedial teaching.
Contraindications:
Piracetam is contraindicated in:
Hypersensitivity to piracetam, other pyrrolidone derivatives or any of the excipients.
- Patients with end-stage renal disease (renal creatinine clearance of less than 20 ml per minute).
- Patients with cerebral haemorrhage.
- Patients suffering from Huntington’s Chorea.
Warning:
Effects on platelet aggregation:
Due to the effect of piracetam on platelet aggregation, caution is recommended in patients with severe haemorrhage, patients at risk of bleeding such as gastrointestinal ulcer, patients with underlying disorders of haemostasis, patients with history of haemorrhagic
CVA (cerebral vascular accident), patients undergoing major surgery including dental surgery, and patients using anticoagulants or platelet anti – aggregant drugs including low dose aspirin.
Renal insufficiency:
Piracetam is eliminated via the kidneys and care should thus be taken in cases of renal insufficiency. Elderly:
For long-term treatment in the elderly, regular evaluation of the creatinine clearance is required to allow dosage adaptation if needed.
Discontinuation:
Abrupt discontinuation of treatment should be avoided as this may induce myoclonic or generalised seizures in some myoclonic patients.
Sickle-cell vaso-occlusive crises:
For sickle-cell indication, a dose lower than 160 mg/kg/day or irregular intake may result in relapse of crises.
Warnings related to the excipients:
– Piracetam, oral solution:
This product contains about 3.5 mmol (or about 80.5 mg) sodium per 24 g piracetam. This should be taken into consideration by patients on a controlled sodium diet.
This medicinal product contains methyl parahydroxybenzoate and propylparahydroxybenzoate which may cause allergic reactions (possibly delayed).
This medicinal product contains glycerol which may cause headache, stomach upset and diarrhea.
SYSTEM CERTIFICATION
1006 OSI
900%
SGS
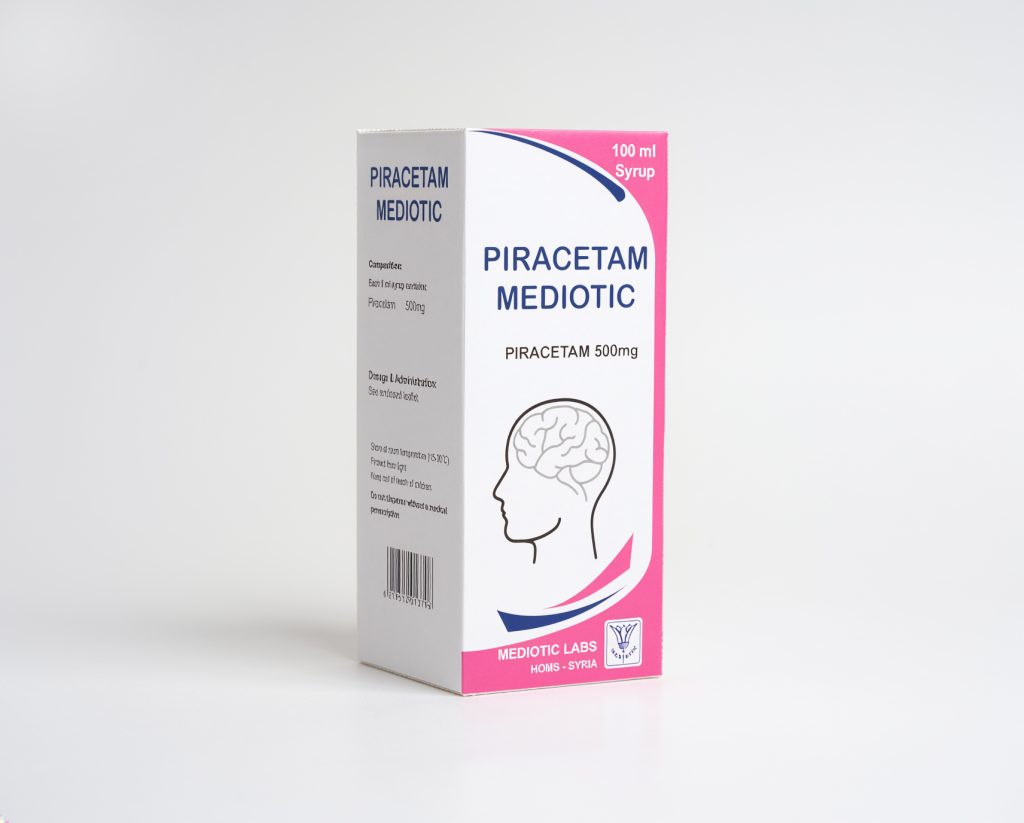
Piracetam Mediotic
oral solution(500mg/5ml)
Chemical Composition: Eash 5ml of solution contains: 500mg piracetam. Excipients: Glycerol, methyl para hydroxybenzoate, propyl para hydroxyl benzoate, sodium acetate, aceticacid, and purified water.
Mechanism of Action:
Piracetam improves membrane stability, allowing the membrane and transmembrane proteins to maintain or recover the three–dimensional structure to exert their function. Piracetam has neuronal and vascular effects. Pharmacokinetics:
Absorption:
Piracetam is rapidly and extensively absorbed following oral administration. In fasted subjects, the peak plasma concentrations are achieved 1 hour after dosing. The absolute bioavailability of piracetam oral formulations is close to 100%. Food does not affect the extent of absorption of piracetam.
Distribution:
Piracetam is not bound to plasma proteins and its volume of distribution is approximately 0.6 l/kg. Piracetam crosses the blood brain barrier as it has been measured in cerebrospinal fluid following intravenous administration.
Metabolism:
Piracetam is not known to be metabolised in the human body. This lack of metabolism is supported by the lengthy plasma half–life in anuric patients and the high recovery of parent compound in urine.
Elimination:
The plasma half–life of piracetam in adults is about 5 hours following either intravenous or oral administration.
The apparent total body clearance is 80-90 ml/min. The major route of excretion is via urine, accounting for 80 to 100% of the dose. Piracetam is excreted by glomerular filtration.
Indications:
Studies carried out in the elderly suffering from loss of memory, vertigo, a lack of concentration or of alertness, changes of mood, a deterioration in behaviour and personal negligence, demonstrate an improvement in symptoms.
These symptoms can also provide an early warning of the onset of pathological ageing such as Alzheimer’s Disease, an Alzheimer type of senile dementia, or the dementia produced by multiple cerebral infarcts. Piracetam is advocated in the treatment of sickle–cell vaso–occlusive crises.
Studies have shown some improvement in children with learning difficulties associated with the written word, particularly with textual understanding which cannot be explained by intellectual backwardness, inadequate education or by the family environment. The administration of Piracetam does not replace other measures also well adapted to correct these learning difficulties, such as remedial teaching.
Contraindications:
Piracetam is contraindicated in:
Hypersensitivity to piracetam, other pyrrolidone derivatives or any of the excipients.
- Patients with end–stage renal disease (renal creatinine clearance of less than 20 ml per minute).
- Patients with cerebral haemorrhage.
- Patients suffering from Huntington’s Chorea.
Warning:
Effects on platelet aggregation:
Due to the effect of piracetam on platelet aggregation, caution is recommended in patients with severe haemorrhage, patients at risk of bleeding such as gastrointestinal ulcer, patients with underlying disorders of haemostasis, patients with history of haemorrhagic CVA (cerebral vascular accident), patients undergoing major surgery including dental surgery, and patients using anticoagulants or platelet anti–aggregant drugs including low dose aspirin.
Renal insufficiency:
Piracetam is eliminated via the kidneys and care should thus be taken in cases of renal insufficiency. Elderly:
For long–term treatment in the elderly, regular evaluation of the creatinine clearance is required to allow dosage adaptation if needed.
Discontinuation:
Abrupt discontinuation of treatment should be avoided as this may induce myoclonic or generalised seizures in some myoclonic patients.
Sickle–cell vaso–occlusive crises:
For sickle–cell indication, a dose lower than 160 mg/kg/day or irregular intake may result in relapse of crises.
Warnings related to the excipients:
– Piracetam, oral solution:
This product contains about 3.5 mmol (or about 80.5 mg) sodium per 24 g piracetam. This should be taken into consideration by patients on a controlled sodium diet.
This medicinal product contains methyl parahydroxybenzoate and propylparahydroxybenzoate which may cause allergic reactions (possibly delayed).
This medicinal product contains glycerol which may cause headache, stomach upset and diarrhea.
Interactions:
Pharmacokinetic interactions :
The drug interaction potential resulting in changes of piracetam pharmacokinetics is expected to be low because approximately 90% of the dose of piracetam is excreted in the urine as unchanged drug. Metabolic interaction of piracetam with other drugs is unlikely. Thyroid hormones:
Confusion, irritability and sleep disorder have been reported during concomitant treatment with thyroid extract (T3 + T4).
The addition of piracetam 9.6 g/d significantly decreased platelet aggregation, B–thromboglobulin release, levels of fibrinogen and von Willebrand’s factors (VIII: C; VIII: vW: Ag; VIII: vW: RCO) and whole blood and plasma viscosity.
Pregnancy and lactation:
Piracetam should not be used during pregnancy unless clearly necessary, when benefit exceeds the risks and the clinical condition of the pregnant mother requires treatment with piracetam.
Piracetam crosses the placental barrier. Drug levels in the newborn are approximately 70% to 90% of maternal levels. Side effects:
Common: nervousness, hyperkinesias, weight increased.
Uncommon: depression, somnolence, asthenia.
Not known: haemorrhagic disorder, anaphylactoid reaction, hypersensitivity, agitation, anxiety, confusion, hallucination, ataxia, balance impaired, epilepsy aggravated, headache, insomnia, vertigo, abdominal pain, abdominal pain upper, diarrhoea, nausea, vomiting, angioneurotic oedema, dermatitis, pruritus, urticaria.
DOSAGE AND ADMINISTRATION:
The total daily dose can range from 30 to 160 mg/kg/day depending on the indication. This is administered twice daily, but may also
be given in three or four separate doses.
– When treating severe symptoms, 12 g daily may need to be administered as an intravenous infusion.
– piracetam, as a long–term therapy for psycho–organic syndrome in the elderly is given in doses ranging from 1.2 to 2.4 g daily, according to the severity of the symptoms. The loading dose can be as high as 4.8 g/day during the initial weeks of treatment. – When treating sickle–cell vaso–occlusive crises, the dose administered is 160 mg/kg/day divided in four equal doses.
– In the treatment of 8 to 13 year–old children with learning difficulties NOOTROPIL is given at a total dose of 3.3 g daily. This is administered either as 8 ml of a 20% solution or 5 ml of a 33% solution twice a day i.e. before breakfast and before the evening meal. The medication may be more easily accepted if given in fruit juice, or in some other drink. Treatment should be continued throughout the school year. The efficacy of a longer period of treatment has not yet been investigated. Elderly:
Adjustment of the dose is recommended in elderly patients with compromised renal function .For long term treatment in the elderly, regular evaluation of the creatinine clearance is required to allow dosage adaptation if needed.
Renal impairment:
Piracetam is contraindicated in severe renal impairment (renal creatinine clearance of less than 20 ml per minute). The daily dose must be individualized according to renal function, an estimate of the patient’s creatinine clearance (CL) in ml/min is needed
Creatinine Clearance (ml/min)
Posology and frequency
usual daily dose, 2 to 4 divided doses
2/3 usual daily dose, 2 or 3 divided doses
Normal Mild
Moderate Severe
End–stage renal disease Overdosage:
> 80
50-79
30-49
1/3 usual daily dose, 2 divided doses
< 30
1/6 usual daily dose, 1 single intake Contraindicated
No additional adverse events specifically related to overdose have been reported with piracetam.
The highest reported overdose with piracetam was oral intake of 75 g where in bloody diarrhoea with abdominal pain, was most probably related to the extreme high dose of sorbitol contained in the used formulation. Treatment
In acute, significant overdosage, the stomach may be emptied by gastric lavage or by induction of emesis. There is no specific antidote for overdose with piracetam. Treatment for an overdose will be symptomatic treatment and may include haemodialysis. The extraction efficiency of the dialyses is 50 to 60% for piracetam.
Storage condition:“store at room temperature, 15°-30°C, away from light” “Keep out of reach of children“.
Packaging: Glass bottle of 100 ml/carton box with Measuring plastic Cup.
* THIS IS A MEDICAMENT *
– Keep out of reach of children.
– A medicament is a product which affects your health, and its consumption
contrary to instructions is dangerous for you.
– Follow strictly doctor’s prescriptions, the method of use and instructions of
the pharmacist who sold the medicament.
– The doctor and pharmacist are experts in medicine, its benefits and risks.
– Do not by yourself interrupt the period of treatment prescribed for you. -Do not repeat the same prescription without consulting your doctor.