COMPOSITION:
Each MEDIOFURYL capsule contains: 200 mg Nifuroxazide.
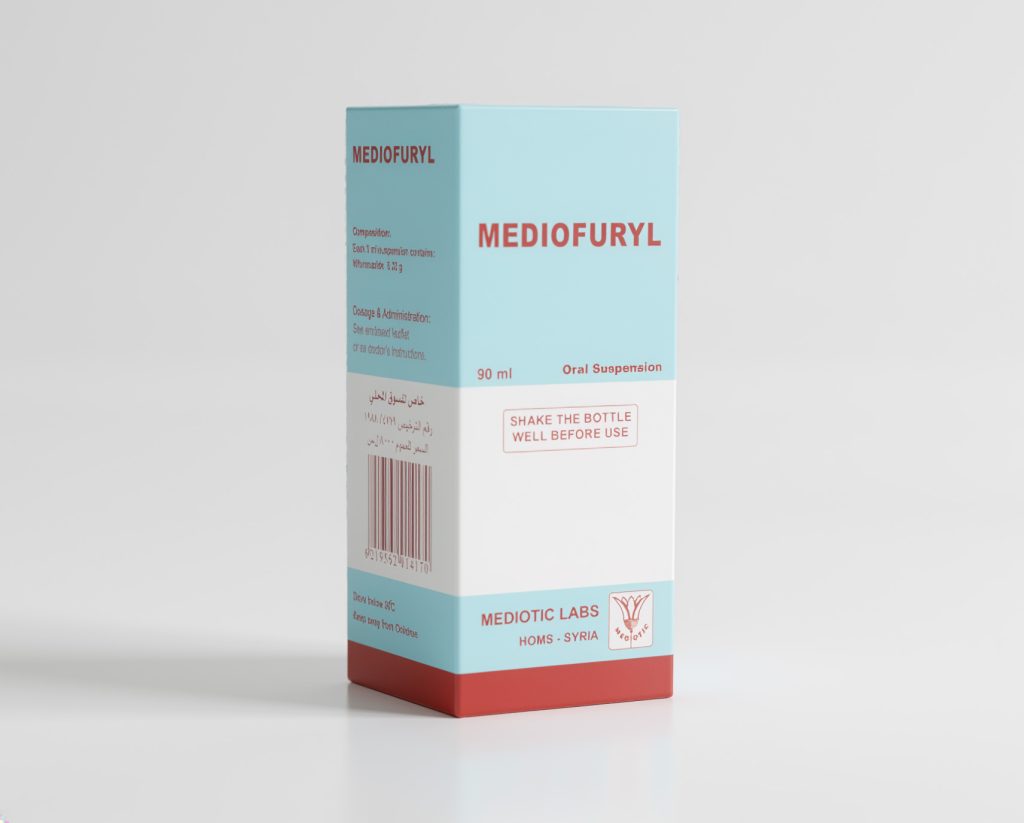
Each 5 mL MEDIOFURYL oral suspension contains: 220 mg Nifuroxazide.
Excipients:
Capsules: Magnesium Stearate, Povidone, Deionized Water.
Oral Suspension: Carboxy Methyl Cellulose, Microcrystalline Cellulose, Citric Acid, Ethyl Alcohol, Antifoaming Emulsion, Banana Flavor, Sucrose, Methyl Paraben Sodium, Propyl Paraben Sodium, Deionized Water. PROPERTIES:
Nifuroxazide, a nitrofurane derivative, is a broad-spectrum intestinal anti-infective agent. Nifuroxazide has been shown to be active against the following pathogens involved in infectious diarrhea:
Escherichia coli,Staphylococcus saprophyticus, Streptococcus, Enterococcus, Bacteroides, Klebsiela, Enterobacter and Serratia.
PHARMACOKINETIC PROPERTIES:
The absorption is extremely low when the intestinal mucosa is not impaired
INDICATIONS:
MEDIOFURYL is indicated in the following conditions:
-Treatment of acute non-invasive diarrhea presumed to be of bacterial origin.
-Treatment and prevention of traveler’s diarrhea.
-Adjunctive therapy for acute inflammatory colitis.
The importance of rehydration by oral rehydration therapy or intravenously should be adjusted according to intensity of the diarrhea, the age and the patients characteristics (associated diseases,…).
CONTRAINDICATIONS:
MEDIOFURYL is contraindicated in:
Hypersensitivity to any of the components or to other nitrofurane derivatives.
Capsule: Children under 15 years of age.
ADVERSE REACTIONS:
This drug is usually well tolerated. However, there is a possibility of allergic reactions to type of rash, urticaria, angioedema or anaphylaxis.
WARNINGS AND PRECAUTIONS:
– Inform your doctor if diarrhea persists more than 2 days or worsens, or if you have fever and/ or blood in the stool. Fluid and electrolyte depletion often occur in patients who have severe diarrhea. In such cases, administration of appropriate fluid and electrolytes is very important. The use of this drug does not preclude the need for appropriate fluid and electrolyte therapy.
–Rehydration is the key treatment of acute diarrhea in children under 2 years. Beyond this age, it should be systematically considered.
-If after 3 days of treatment the diarrhea persists. What to do should be reassessed and the need for oral rehydration or intravenous should be considered.
-In case of infective diarrhea with clinical symptoms suggesting an invasive phenomenon, use of antibacterials good to systemic dissemination.
-Due to the presence of sucrose, patients with rare hereditary problems of fructose intolerance, glucose-galactose malabsorption or sucrase-isomaltase insufficiency should not take this medicine.
-In the event of greater than 6 loose stools per day or diarrhea lasting for more than 24 hours or is accompanied by a loss of weight, or in case of presence of blood or mucus in the stool, the patient should consult his doctor
-The patient should be informed to exclude certain inputs, particularly raw vegetables, fruits, green vegetables, spicy dishes, as well as frozen food or beverages, and to focus on grilled meats, rice.
DRUG INTERACTIONS:
This medication cannot be taken with drugs that induce an antabuse reaction and CNS depressants.
Pregnancy and breast-feeding:
As a precaution, it is best not to use nifuroxazide during pregnancy.
Breast-feeding remains possible in case of brief treatment with this drug.
OVERDOSE:
In case of overdose of nifuroxazide, a patient monitoring must be carried out and symptomatic treatment should be implemented.
DOSAGE AND ADMINISTRATION:
MEDIOFURYL is for oral use; swallow the capsule with a glass of water.
-Take MEDIOFURYL as directed by your doctor.
-The duration of treatment is limited to 3 days.
-In case of a missed dose: Take the missed dose as soon as you remember, unless the next dose is near. Do not take a double dose at once.
– don’t give under 18 years.
Capsule:
The usual recommended dose for adults is 1 capsule of 200 mg, 4 times per day. Or 1 capsule of 400 mg 2 times per day. STORAGE CONDITIONS:
Protect from light and moisture.
Store at room temperature, between (15-30)°C.
Keep out of reach of children.
PACKAGING:
3 blisters, each contains 10 capsules/carton box.
Glass bottle of 90 mL oral suspension/carton box, with a metallic screw cap.