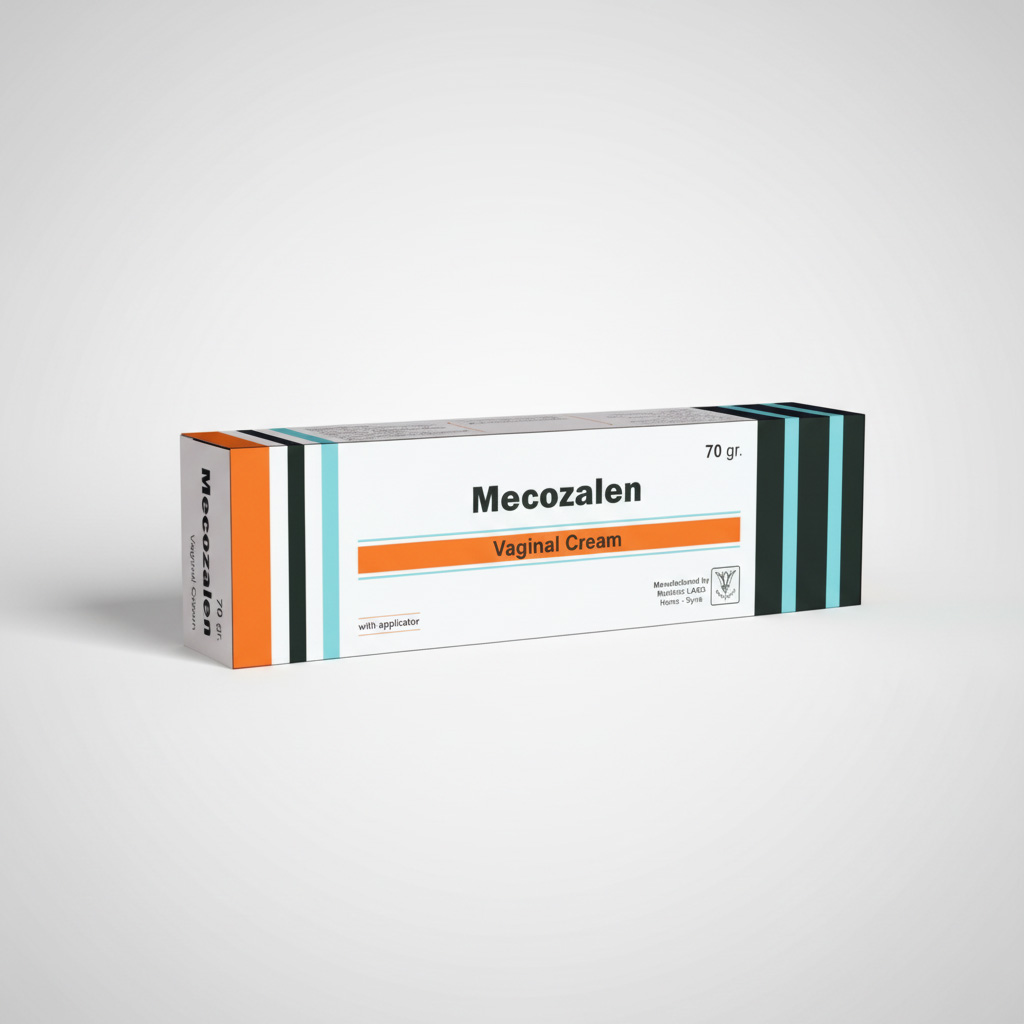
Chemical Composition: Each vaginal Suppository contains Metronidazol 500 mg, Miconazole Nitrate 100mg.
Excipients: Hydrogenated vegetable oil,Aerosil.
PHARMACODYNAMICS:
This product contains miconazole for antifungal, metronidazole for antibacterial and antitrichomonal effects. Miconazole nitrate which is an antifungal has a wide spectrum of activity and is particularly effective against pathogen fungi including Candida albicans. In addition, miconazole nitrate is effective against Gram (+) bacteria. Metronidazole is an antibacterial and antiprotozoal agent that is effective against Gardenella vaginalis and against anaerobic bacteria including anaerobic streptococci and Trichomonas vaginalis.
PHARMACOKINETICS:
Absorption of miconazole nitrate through the intravaginal route is very low (approximately 1.4% of dose). Bioavailability of metronidazole by this route is 20% compared to the oral route. Miconazole nitrate cannot be detected in plasma after vaginal application of the suppository. Steady state level of metronidazole in plasma reaches 1.6 – 7.2 μg/ml. Metronidazole is metabolized in the liver. The hydroxyl- metabolite is effective. The half-life of metronidazole is 6- 11 hours. Approximately 20% of the dose is excreted unchanged in urine.
INDICATIONS:
Metronidazole + Miconazole nitrate vaginal suppository is used for local treatment of vaginalis infection with the involvement of certain fungi (eg Candida albicans), some bacteria and other pathogenic organisms (Trichomonas vaginalis). CONTRAINDICATIONS:
Metronidazole + Miconazole nitrate should not be used in the following cases: Hypersensitivity to any of the ingredients of the suppository.
Pregnancy (particularly in the first 3 months).
Severe problems with liver (liver function disorders including metabolic disease called “porphyria”.
Diseases of the nervous system.
Disturbances in the production of blood.
WARNINGS / PRECAUTIONS:
Alcohol should not be taken during the therapy and for at least 24-48 hours after the end of course of treatment because of the possibility of increased alcohol incompatibility.
The suppository should not be used together with the contraceptive diaphragms and condoms since the ovule base may affect the rubber, which may possibly break. Pregnancy and Lactation:
After the first three months of pregnancy, Metronidazole + Miconazole nitrate suppository can be used when considered essential by the doctor, but must be used under observation.
Breastfeeding is not allowed while using Metronidazole + Miconazole nitrate, since active ingredients pass into the milk. Breastfeeding may be started again 24-48 hours after the treatment is finished.
SIDE EFFECTS:
Vaginal Disorders: Vaginal irritation (burning, itching). Owing to the inflammation of the vaginal mucosa in vaginitis, vaginal irritation can be seen after the administration of the first suppository or towards the 3rd day of the treatment. These complaints disappear quickly when the treatment continues. If there is severe irritation, the physician should be informed because it may be necessary to stop the treatment.
Vaginal products should be avoided
Check with the doctor before use if vaginal yeast infections are frequent (such as once a
month or 3 in 6 months), it could be serious underlying medical cause, including (diabetes or a weakened immune system) or if the vaginal itching and discomfort is for the first time. Stop use and check with the doctor if
Symptoms do not get better in 3 days
Symptoms last more than 7 days
If the patient gets a rash or hives, abdominal pain, fever, chills, nausea, vomiting, or foul- smelling vaginal discharge, it could be more serious condition.
Laboratory Tests:
If there is a lack of response to Miconazole Vaginal Suppositories, appropriate microbiological studies should be repeated to confirm the diagnosis and rule out other pathogens. DRUG INTERACTION:
Miconazole administered systemically is known to inhibit CYP3A4/2C9. Due to the limited systemic availability after vaginal application, clinically interactions occur very rarely.
In patients on oral anticoagulants, such as warfarin, caution should be exercised and anticoagulant effect should be monitored because bleeding or bruising may occur
The effects and side effects of other drugs metabolized by CYP2C9 (e.g. oral hypoglycemics and phenytoin) and also CYP3A4 (e.g., simvastatin and lovastatin and calcium channel blockers such as dihydropyridines and verapamil), when co-administered with miconazole, can be increased and caution should be exercised.
Pregnancy and lactation:
Pregnancy: Although intravaginal absorption is limited, miconazole should be used in the first trimester of pregnancy only if the potential benefits outweigh the possible risks.
Breastfeeding: It is not known whether miconazole nitrate is excreted in human milk. Caution should be exercised when using miconazole during breastfeeding.
ADVERSE REACTIONS:
Very common: Genital pruritus, vaginal burning sensation, vulvovaginal discomfort.
Common: Rash, Dysmenorrhea.
Uncommon: pruritic rash, urticaria, headaches, itching or irritation, cramping.
Rare: hives.
OVERDOSE:
Symptoms: miconazole vaginal cream and ovules are intended for local application and not for oral use. In case of accidental ingestion, no problems are expected.
Treatment: In the event of accidental ingestion of large quantities, appropriate supportive care should be used.
STORAGE CONDITIONS:
Vaginal cream: Store at temperature between (15-25) °C
Vaginal suppositories: Store at temperature between (15-25) °C.
Keep out of reach of children.
PACKAGE:
Vaginal cream:
Aluminium tube contains 70 g MECOZALEN vaginal cream with an applicator/carton box. Vaginal Suppositories:
Carton MECOZALEN box contains 3 vaginal Suppositories.
Carton MECOZALEN box contains 6 vaginal Suppositories.