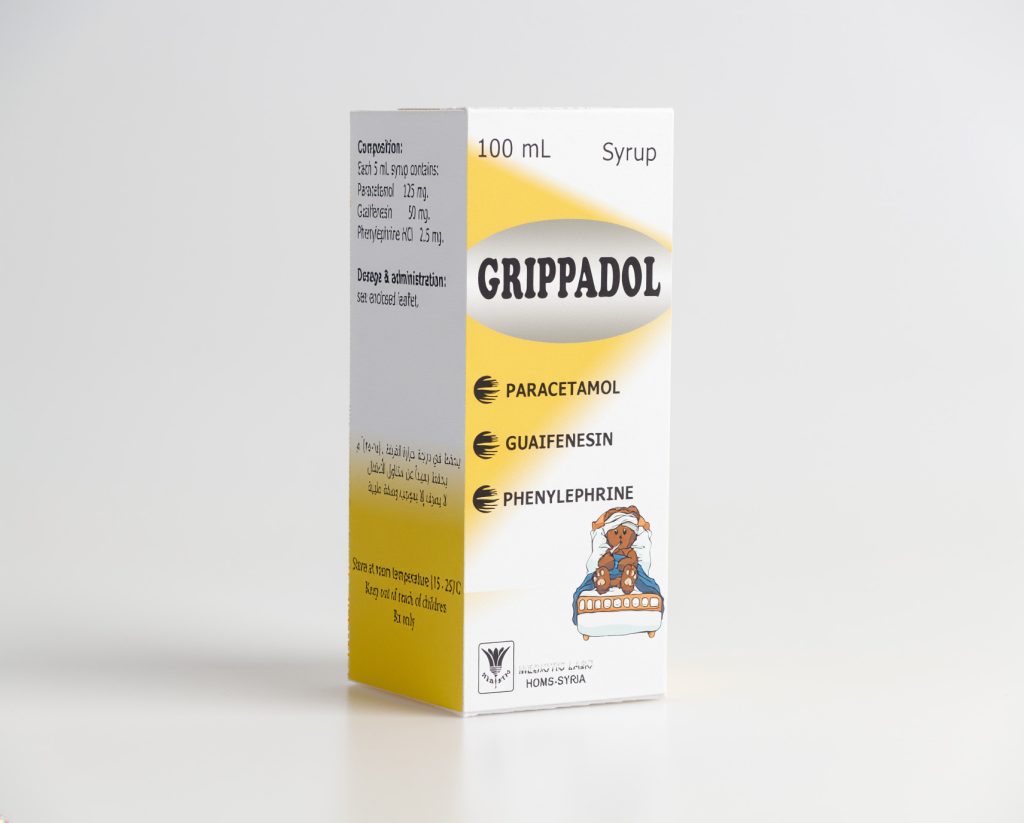
GRIPPADOL (syrup)
125 mg paracetamol, phenylephrine HCI 2.5 mg, Guaifenesin 50 mg / 5 ml
Composition and Excipients: Each 5 ml of the syrup contains:
Paracetamol 125 mg, Phenylephrine HCI 2.5 mg, Guafenesin 50 mg.
Excipients: Sorbitol 70%, Glycerin, Alcohol 96%, Propylene glycol, Sodium saccharine, Acesulfame potassium, Sodium citrate, Xanthan gum, Citric acid monohydrate, Mint flavor, Sunset yellow, Patent Blue, Purified water.
Properties:
Paracetamol is an analgesic and antipyretic.
Guaifenesin is an expectorant.
Phenylephrine Hydrochloride is a sympathomimetic decongestant.
Pharmacokinetic properties:
Paracetamol is readily absorbed from the gastrointestinal tract, it is metabolised in the liver and excreted in the urine.
Guaifenesin is rapidly absorbed after oral administration, it is rapidly metabolised and excreted in the urine.
Phenylephrine hydrochloride is irregularly absorbed from the gastrointestinal tract and undergoes first-pass metabolism by monoamine oxidase in the gut and liver; orally administered phenylephrine has reduced bioavailability, it is excreted in the urine almost entirely.
Indications:
Short term symptomatic relief of colds, chills and influenza including chesty coughs. Contraindications:
- Hypersensitivity to paracetamol and/or other constituents.
- Concomitant use of other sympathomimetic decongestants.
- Phaeochromocytoma.
- Closed angle glaucoma.
- Hepatic or severe renal impairment, hypertension, hyperthyroidism, diabetes, heart disease or those taking tricyclic antidepressants or beta-blocking drugs and those patients who are taking or have taken, within the last two weeks, monoamine oxidase inhibitors.
- Children under 6 years of age
Warnings and precautions:
Duration of therapy should not exceed 5 days
- Care is advised in the administration of paracetamol to patients with severe renal or severe hepatic impairment. The hazards of overdose are greater in those with (non-cirrhotic) alcoholic liver disease.
- Patients suffering from chronic cough or asthma should consult a physician before taking this product.
- Patients should stop using the product and consult a health care professional if cough lasts for more than 5 days or comes back, or is accompanied by a fever, rash or persistent headache.
- Medical advice should be sought before taking this product in patients with these conditions: o An enlargement of the prostate gland
o Occlusive vascular disease (e.g. Raynaud’s Phenomenon)
o Cardiovascular disease
- This product should not be used by patients taking other sympathomimetics (such as decongestants, appetite suppressants and amphetamine-like psychostimulants)
- Concomitant use of other paracetamol-containing products should be avoided. If symptoms persist the doctor should be consulted.
- It should not be taken with other flu, cold or decongestant products or a cough suppressant. Drug Interaction:
- If urine is collected within 24 hours of a dose of this product, a metabolite may cause a color interference with laboratory determinations of 5 hydroxyindoleacetic acid (5-HIAA) and vanillymandelic acid (VMA).
Paracetamol:
- The anticoagulant effect of warfarin and other coumarins may be enhanced by prolonged regular use of paracetamol with increased risk of bleeding; occasional doses have no significant effect.
- The hepato-toxicity of paracetamol may be potentiated by excessive intake of alcohol.
- The speed of absorption of paracetamol may be increased by metoclopramide or domperidone and absorption reduced by colestyramine.
Phenylephrine: It should be used with caution in combination with the following drugs as interactions have been reported:
- Monoamine oxidase inhibitors: Hypertensive interactions occur between sympathomimetic amines such as phenylephrine and monoamine oxidase inhibitors.
Sympathomimetic amines: Concomitant use of phenylephrine with other sympathomimetic amines can increase the risk of cardiovascular side effects.
- Beta-blockers and other antihypertensives: Phenylephrine may reduce the efficacy of beta- blocking drugs and antihypertensive drugs. The risk of hypertension and other cardiovascular side effects may be increased.
-
- Tricyclic antidepressants: May increase the risk of cardiovascular side effects with phenylephrine.
- Ergot alkaloids (ergotamine and methylsergide): Increased risk of ergotism.
- Digoxin and cardiac glycosides: Increase the risk of irregular heartbeat or heart attack. Pregnancy and lactation:
-This product should not be used during pregnancy without medical advice. The safety of guaiphenesin and phenylephrine during pregnancy has not been established.
-Paracetamol and phenylephrine are excreted in breast milk but not in a clinically significant amount. This product should not be used whilst breast feeding without medical advice. Effects on ability to drive and use machines:
Patients should be advised not to drive or operate machinery if affected by dizziness.
- Side effects:Paracetamol: Due to limited clinical trial data, the frequency of these adverse events is not known. Events reported from extensive post-marketing experience at therapeutic/labelled dose are the following: Thrombocytopenia, Agranulocytosis, Anaphylaxis, Cutaneous hypersensitivity reactions including (skin rashes, angiodema and Stevens Johnson syndrome, toxic epidermal necrolysis), Bronchospasm( There have been cases of bronchospasm with paracetamol, but these are more likely in asthmatics sensitive to aspirin or other NSAIDs), Hepatic dysfunction, Acute pancreatitis.Phenylephrine: The following adverse events have been observed in clinical trials and may therefore represent the most commonly occurring adverse events: Nervousness, irritability, restlessness, and excitability, Headache, dizziness, insomnia, increased blood pressure, Nausea, Vomiting, diarrhea.
Guaifenesin: The frequency of the following events is unknown but considered likely to be rare: Allergic reactions, angioedema, anaphylactic reactions, Dyspnoea, Nausea, vomiting, abdominal discomfort, Rash, urticaria
Dosage and Administration:
Duration of therapy should not exceed 5 days.
For 125 mg paracetamol, phenylephrine hcl 2.5 mg, Guaifenesin 50 mg/5 ml:
Adults and children 12 years and over: four 5 ml spoonfuls. Repeat every four hours as necessary. Do not exceed four doses per 24 hours.
Children 6-12 years of age: should not be given except on medical advice.
Children under 6 years: contraindicated.
Elderly: The normal adult dose may be taken.
Overdose:
Immediate medical advice should be sought in the event of an overdose, because of the risk of delayed, serious liver damage
Paracetamol: Liver damage is possible in adults who have taken 10g or more of paracetamol. Ingestion of 5g or more of paracetamol may lead to liver damage if the patient has risk factors (If the patient, Is on long term treatment with (carbamazepine, phenobarbitone, phenytoin, primidone, rifampicin, St John’s Wort or other drugs that induce liver enzymes), Regularly consumes ethanol in excess of recommended amounts or Is likely to be glutathione deplete e.g. eating disorders, cystic fibrosis, HIV infection, starvation, cachexia).
Symptoms of paracetamol overdosage in the first 24 hours are pallor, nausea, vomiting, anorexia and abdominal pain. Liver damage may become apparent 12 to 48 hours after ingestion. In severe poisoning, hepatic failure may progress to encephalopathy, haemorrhage, hypoglycaemia, cerebral oedema, and death.
Acute renal failure with acute tubular necrosis strongly suggested by loin pain, hematuria and proteinuria may develop even in the absence of severe liver damage. Cardiac arrhythmias and pancreatitis have been reported.
Immediate treatment is essential in the management of paracetamol overdose. Despite a lack of significant early symptoms, patients should be referred to hospital urgently for immediate medical attention. Symptoms may be limited and may not reflect the severity of overdose or the risk of organ damage. Treatment with activated charcoal should be considered if the overdose has been taken within 1 hour. Treatment with N-acetylcysteine may be used up to 24 hours after ingestion of paracetamol. The effectiveness of the antidote declines sharply after 8 hours.
Phenylephrine: Phenylephrine overdosage is likely to result in effects similar to those listed under adverse reactions. Additional symptoms may include hypertension and reflux bradycardia. In severe cases confusion, hallucinations, seizures, and arrhythmia may occur. Treatment should be as clinically appropriate. Severe hypertension may need to be treated with an alpha blocking drug such as phentolamine.
Guaifenesin: Very large doses of guaifenesin cause nausea and vomiting. Vomiting would be treated by fluid replacement and monitoring of electrolytes.
Storage conditions:
Store at room temperature, (15–25) °C.
Packaging:
Glass bottle of 100 mL/carton box, with a measured cap.