Excipients: Sucrose,polysorbate80,sodium benzoate, sodium carboxymethyl cellulose, methyl paraben sodium, Propyl paraben sodium,Aspartame, Sodium Saccharine, Colloidal silicon dioxide, Disodium edetate ,Sodium chloride, Limon flavor, Orange flavor, xanthan gum,Menthol, and purified water.
Mechanisn of action: Ofloxacin is a quinolone antimicrobial agent. The mechanism of action of ofloxacin and other fluoroquinolone antimicrobials involves inhibition of bacterial topoisomerase IV and DNA gyrase (both of which are type II topoisomerases) enzymes required for DNA replication, transcription, repair and recombination. Ofloxacin has in vitro activity against a wide range of Gram-negative and Gram-positive microorganisms. Ofloxacin is often bactericidal at concentrations equal to or slightly greater than inhibitory concentrations.
Ornidazole is a 5-nitroimidazole derivative active against protozoa and anaerobic bacteria. It is converted to reduction products that interact with DNA to cause destruction of the helical DNA structure and strand, leading to a protein synthesis inhibition and cell death in susceptible organisms. PHARMACOKINETICS:
Ofloxacin: Following oral administration, the bioavailability of ofloxacin in the tablet formulation is approximately 98%. Maximum serum concentrations are achieved 1-2 hours after an oral dose. Absorption of ofloxacin after single or multiple doses of 200-400 mg is predictable, and the amount of drug absorbed increases proportionately with the dose. Ofloxacin has biphasic elimination. Following multiple oral doses at steady-state administration, the half-lives are approximately 4-5 hours and 20-25 hours. Elimination is mainly by renal excretion.
Ornidazole: Following oral administration ornidazole is rapidly absorbed. Mean absorption is 90%. Peak plasma concentrations are reached within 3 hours. The mean volume of distribution after intravenous administration is 1 litre per kg. Plasma protein-binding of ornidazole is about 13%. Ornidazole is mainly metabolised to 2-hydroxymethyl and a-hydroxymethyl metabolites in the liver. The half-life is about 13 hours. While 85% of a single dose is eliminated within the first 5 days (most of this being metabolised), 4% of the dose is excreted as unaltered substance in the urine.
INDICATIONS:
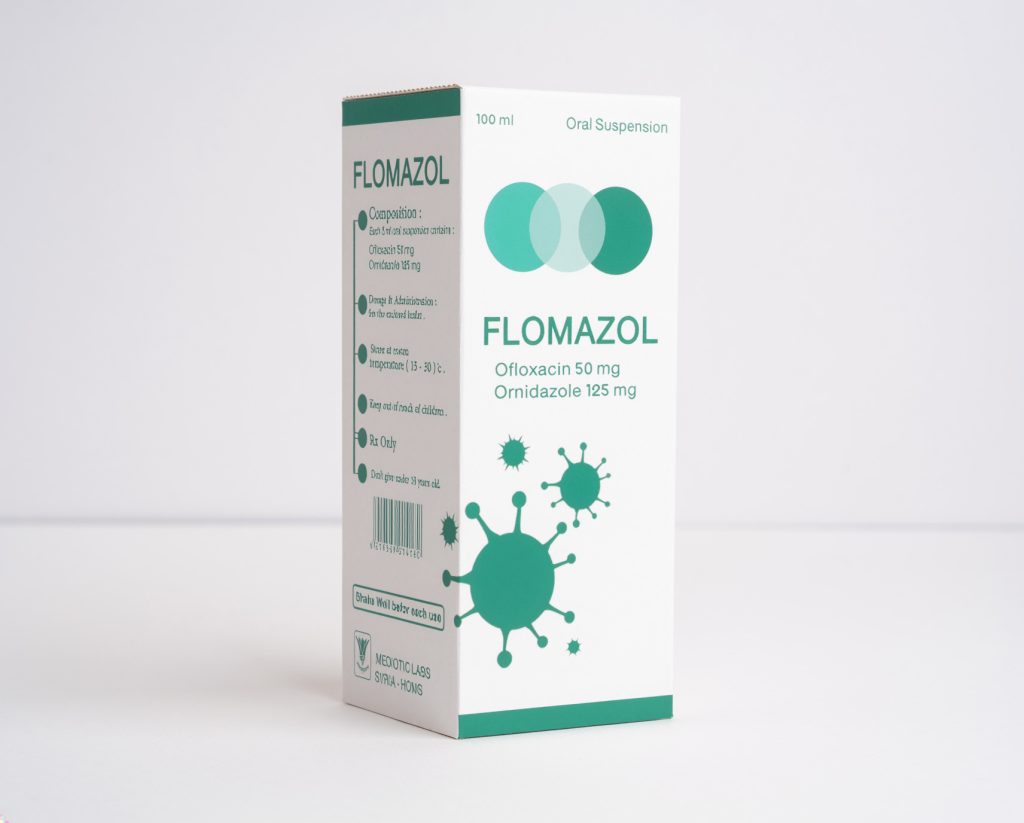
Flomazol are indicated for the treatment of diarrhea of mixed infection in adults only. CONTRAINDICATIONS:
flomazol are contraindicated in people with a history of hypersensitivity associated with the use of ofloxacin, ornidazole or any member of the quinolone or nitroimidazole group of antimicrobial agents. WARNING AND PRECAUTIONS:
Ofloxacin:
Fluoroquinolones, including ofloxacin, have been associated with disabling and potentially irreversible serious adverse reactions from different body systems that can occur together in the same patient. Commonly seen adverse reactions include tendinitis, tendon rupture, arthralgia, myalgia, peripheral neuropathy and CNS effects (hallucinations, anxiety, depression, insomnia, severe headaches and confusion). These reactions can occur within hours to weeks after starting ofloxacin. Discontinue ofloxacin immediately at the first signs or symptoms of any serious adverse reaction. In addition, avoid the use of fluoroquinolones, including ofloxacin, in patients who have experienced any of these serious adverse reactions associated with fluoroquinolones. Tendinitis and Tendon Rupture:
Fluoroquinolones, including ofloxacin, have been associated with an increased risk of tendinitis and tendon rupture in all ages. This adverse reaction most frequently involves the Achilles tendon and rupture of the Achilles tendon, and has also been reported with the rotator cuff (the shoulder), the hand, the biceps, the thumb, and other tendons. Tendinitis or tendon rupture can occur within hours or days of starting ofloxacin, or as long as several months after completion of fluoroquinolone therapy. Tendinitis and tendon rupture can occur bilaterally. The risk of developing fluoroquinolone-associated tendinitis and tendon rupture is increased in patients over 60 years of age, in those taking corticosteroid drugs, and in patients with kidney, heart or lung transplants. Other factors that may independently increase the risk of tendon rupture include strenuous physical activity, renal failure, and previous tendon disorders such as rheumatoid arthritis. Tendinitis and tendon rupture have been reported in patients taking fluoroquinolones who do not have the above risk factors. Discontinue ofloxacin immediately if the patient experiences pain, swelling, inflammation or rupture of a tendon. Avoid fluoroquinolones, including ofloxacin, in patients who have a history of tendon disorders or have experienced tendinitis or tendon rupture. Patients should be advised to rest at the first sign of tendinitis or tendon rupture, and to contact their healthcare provider regarding changing to a non-quinolone antimicrobial drug.
Peripheral Neuropathy:
Fluoroquinolones, including ofloxacin, have been associated with an increased risk of peripheral neuropathy. Cases of sensory or sensorimotor axonal polyneuropathy affecting small and/or large axons, resulting in paraesthesia, hypoesthesia, dysaesthesia and weakness, have been reported in patients receiving fluoroquinolones, including ofloxacin. Symptoms may occur soon after initiation of norfloxacin and may be irreversible in some patients. Discontinue ofloxacin immediately if the patient experiences symptoms of peripheral neuropathy, including pain, burning, tingling, numbness and/or weakness, or other alterations in sensations, including light touch, pain, temperature, position sense and vibratory sensation and/or motor strength in order to minimise the development of an irreversible condition. Avoid fluoroquinolones, including ofloxacin, in patients who have previously experienced peripheral neuropathy. Exacerbation of Myasthenia Gravis:
Fluoroquinolones, including ofloxacin, have neuromuscular-blocking activity and may exacerbate muscle weakness in persons with myasthenia gravis.
Avoid ofloxacin in patients with a known history of myasthenia gravis.
CNS Effects:
Fluoroquinolones, including ofloxacin, have been associated with an increased risk of CNS effects, including convulsions, increased intracranial pressure (including pseudotumour cerebri), and toxic psychoses. Quinolones may also cause CNS stimulation, which may lead to tremors, restlessness, lightheadedness, confusion, and hallucinations. If these reactions occur in patients receiving ofloxacin, the drug should be discontinued and appropriate measures instituted.
The effects of ofloxacin on brain function or on the electrical activity of the brain have not been tested. Therefore, until more information becomes available, ofloxacin, like all other quinolones, should be used with caution in patients with known or suspected CNS disorders, such as severe cerebral arteriosclerosis, epilepsy, and other factors that predispose to seizures.
The safety and efficacy of ofloxacin in paediatric patients and adolescents (under the age of 18 years), pregnant women, and lactating women has not been established. Hypersensitivity Reactions:
Serious, and occasionally fatal, hypersensitivity and/or anaphylactic reactions have been reported in patients receiving therapy with quinolones, including ofloxacin. These reactions often occur following the first dose. Some reactions have been accompanied by cardiovascular collapse, hypotension/shock, seizure, loss of consciousness, tingling, angio-oedema (including tongue, laryngeal, throat or facial oedema/swelling), airway obstruction (including bronchospasm, shortness of breath, and acute respiratory distress), dyspnoea, urticaria, itching, and other serious skin reactions. This drug should be discontinued immediately at the first appearance
of a skin rash or any other sign of hypersensitivity. Serious acute hypersensitivity reactions may require treatment with epinephrine and other resuscitative measures, including oxygen, intravenous fluids, antihistamines, corticosteroids, pressor amines, and airway management, as clinically indicated.
The drug should be discontinued immediately at the first appearance of skin rash, jaundice, or any other sign of hypersensitivity and supportive measures instituted.
Clostridium difficile-associated diarrhoea (CDAD) has been reported with the use of nearly all antibacterial agents, including ofloxacin tablets, and may range in severity from mild diarrhoea to fatal colitis.
CDAD must be considered in all patients who present with diarrhoea following antibiotic use. Careful medical history is necessary since CDAD has been reported to occur over 2 months after the administration of antibacterial agents.
If CDAD is suspected or confirmed, ongoing antibiotic use not directed against C. difficile may need to be discontinued. Appropriate fluid and electrolyte management, protein supplementation, antibiotic treatment of C. difficile, and surgical evaluation should be instituted as clinically indicated. Ofloxacin has not been shown to be effective in the treatment of syphilis.
Antimicrobial agents used in high doses for short periods of time to treat gonorrhoea may mask or delay the symptoms of incubating syphilis. All patients with gonorrhoea should have a serologic test for syphilis at the time of diagnosis.
Patients treated with ofloxacin for gonorrhoea should have a follow-up serologic test for syphilis after three months and, if positive, treatment with an appropriate antimicrobial should be instituted.
General:
Prescribing ofloxacin tablets in the absence of a proven or strongly suspected bacterial infection or a prophylactic indication is unlikely to provide benefit to the patient and increases the risk of the development of drug-resistant bacteria.
Adequate hydration of patients receiving ofloxacin should be maintained to prevent the formation of highly concentrated urine.
Administer ofloxacin with caution in the presence of renal or hepatic insufficiency/impairment. In patients with known or suspected renal or hepatic insufficiency/impairment, careful clinical observation and appropriate laboratory studies should be performed prior to and during therapy since elimination of ofloxacin may be reduced. In patients with impaired renal function (creatinine clearance ≤50 mg/mL), alteration of the dosage regimen is necessary.
Drug therapy should be discontinued If photosensitivity/phototoxicity occurs.
As with other quinolones, ofloxacin should be used with caution in any patient with a known or suspected CNS disorder that may predispose to seizures or lower the seizure threshold (e.g. severe cerebral arteriosclerosis, epilepsy) or in the presence of other risk factors that may predispose to seizures or lower the seizure threshold (e.g. certain drug therapy, renal dysfunction).
A possible interaction between oral hypoglycaemic drugs (e.g. glyburide/glibenclamide) or with insulin and fluoroquinolone antimicrobial agents has been reported, resulting in a potentiation of the hypoglycaemic action of these drugs. The mechanism for this interaction is not known. If a hypoglycemic reaction occurs in a patient being treated with ofloxacin, discontinue ofloxacin immediately and consult a physician.
As with any potent drug, periodic assessment of organ system functions, including renal, hepatic, and haematopoietic, is advisable during prolonged therapy.
Torsades de pointes:
Some quinolones, including ofloxacin, have been associated with prolongation of the QT interval on the electrocardiogram and infrequent cases of arrhythmia. Ofloxacin should be avoided in patients with known prolongation of the QT interval, patients with uncorrected hypokalaemia, and patients receiving Class IA (quinidine, procainamide), or Class III (amiodarone, sotalol) anti-arrhythmic agents.
Other Information for Patients
Patients should be advised on the following:
To drink fluids liberally.
That mineral supplements, vitamins with iron or minerals, calcium-, aluminium- or magnesium-based antacids, sucralfate or didanosine tablets or the paediatric powder for oral solution should not be taken within the 2-hour period before or within the 2-hour period after taking ofloxacin.
That ofloxacin can be taken without regard to meals.
That antibacterial drugs, including ofloxacin tablets, should only be used to treat bacterial infections. They do not treat viral infections (e.g. the common cold). When ofloxacin tablets are prescribed to treat a bacterial infection, patients should be told that although it is common to feel better early in the course of therapy, the medication should be taken exactly as directed. Skipping doses or not completing the full course of therapy may (1) decrease the effectiveness of the immediate treatment; and, (2) increase the likelihood that bacteria will develop resistance and will not be treatable by ofloxacin tablets or other antibacterial drugs in the future. Ornidazole:
Caution should be exercised in patients with diseases of the CNS, e.g. epilepsy or multiple sclerosis. The effect of other medicines can be intensified or impaired. PREGNANCY:
Ofloxacin:
Pregnancy Category C, There are, however, no adequate and well-controlled studies in pregnant women. Ofloxacin should be used during pregnancy only if the potential benefit justifies the potential risk to the foetus. Ornidazole:
no controlled studies have been carried out in pregnant women. As a matter of principnle, ornidazole should not be prescribed in early pregnancy or to nursing mothers except when absolutely necessary
NURSING MOTHERS:
Because of the potential for serious adverse reactions from ofloxacin in nursing infants, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
It is not known whether ornidazole is excreted in human milk. In making the decision whether or not to discontinue breastfeeding or whether or not ornidazole treatment should be discontinued/avoided, the benefit of breastfeeding to the infant and the benefit of ornidazole treatment for the nursing mother must be considered.
PEDIATRIC USE:
Safety and effectiveness in paediatric patients and adolescents below the age of 18 years have not been established.
GERIATRIC USE:
Geriatric patients are at increased risk for developing severe tendon disorders, including tendon rupture, when being treated with a fluoroquinolone such as ofloxacin. This risk is further increased in patients receiving concomitant corticosteroid therapy. Caution should be used when prescribing ofloxacin to elderly patients especially those on corticosteroids.
Elderly patients may be more sensitive to drug-associated effects on the QT interval. Therefore, precaution should be taken when using ofloxacin with concomitant drugs that can result in prolongation of the QT interval (e.g. Class IA or Class III anti-arrhythmics) or in patients with risk factors for torsades de pointes (e.g. known QT prolongation, uncorrected hypokalaemia).
Effects on the Ability to Drive and Use Machines:
Ofloxacin;
Since there have been occasional reports of somnolence, impairment of skills, dizziness and visual disturbances, patients should know how they react to ofloxacin before they drive or operate machinery. These effects may be enhanced by alcohol.
Ornidazole:
Somnolence, dizziness, tremor, rigidity, poor coordination, seizures, vertigo or temporary loss of consciousness may occur in patients receiving ornidazole. If they occur, such effects may affect tasks requiring alertness, including the patient’s ability to drive and operate machinery.
DRUG INTERACTIONS:
Ofloxacin:
Antacids, Sucralfate, Metal Cations, Multivitamins:
Quinolones form chelates with alkaline earth and transition metal cations. Administration of quinolones with antacids containing calcium, magnesium or aluminium, with sucralfate, with divalent or trivalent cations such as iron, or with multivitamins containing zinc or with didanosine, or the paediatric powder for oral solution may substantially interfere with the absorption of quinolones, resulting in systemic levels considerably lower than desired. These agents should not be taken within the 2-hour period before or within the 2-hour period after ofloxacin administration.
Caffeine
Interactions between ofloxacin and caffeine have not been detected.
Cimetidine
Cimetidine has demonstrated interference with the elimination of some quinolones. This interference has resulted in significant increases in the half-life and AUC of some quinolones. The potential for interaction between ofloxacin and cimetidine has not been studied.
Cyclosporine
Elevated serum levels of cyclosporine have been reported with concomitant use of cyclosporine with some other quinolones. The potential for interaction between ofloxacin and cyclosporine has not been studied. Drugs Metabolised by Cytochrome P450 Enzymes
Most quinolone antimicrobial drugs inhibit cytochrome P450 enzyme activity. This may result in a prolonged half-life for some drugs that are also metabolised by this system (e.g. cyclosporine,
theophylline/methylxanthines, warfarin) when co-administered with quinolones. The extent of this inhibition varies among different quinolones.
Non-Steroidal Anti-Inflammatory Drugs:
The concomitant administration of a non-steroidal anti-inflammatory drug with a quinolone, including ofloxacin, may increase the risk of CNS stimulation and convulsive seizures.
Probenecid :
The concomitant use of probenecid with certain other quinolones has been reported to affect renal tubular secretion. The effect of probenecid on the elimination of ofloxacin has not been studied. Theophylline:
Steady-state theophylline levels may increase when ofloxacin and theophylline are administered concurrently. As with other quinolones, concomitant administration of ofloxacin may prolong the half-life of theophylline, elevate serum theophylline levels, and increase the risk of theophylline-related adverse reactions. Theophylline levels should be closely monitored and theophylline dosage adjustments made, if appropriate, when ofloxacin is co-administered. Adverse reactions (including seizures) may occur with or without an elevation in the serum theophylline level.
Warfarin:
Some quinolones have been reported to enhance the effects of the oral anticoagulant warfarin or its derivatives. Therefore, if a quinolone antimicrobial is administered concomitantly with warfarin or its derivatives, the prothrombin time or other suitable coagulation test should be closely monitored. Antidiabetic Agents (e.g. Insulin, Glyburide/Glibenclamide)
Since disturbances of blood glucose, including hyperglycaemia and hypoglycaemia, have been reported in patients treated concurrently with quinolones and an antidiabetic agent, careful monitoring of blood glucose is recommended when these agents are used concomitantly.
Ornidazole:
Alcohol must not be ingested when taking ornidazole or for at least 3 days after discontinuing the medicine. Ornidazole potentiates the effect of coumarin type oral anticoagulants. The dosage of the anticoagulant has to be adjusted accordingly. Caution must be exercised when taking ornidazole together with lithium, cimetidine and antiepileptic medicines such as phenytoin and phenobarbital. Ornidazole prolongs the muscle relaxant effect of vecuronium bromide.
ADVERSE REACTIONS:
nausea, insomnia, headache, dizziness, diarrhoea, vomiting, rash, pruritus, external genital pruritus in women, vaginitis, and dysgeusia.
OVERDOSAGE:
Ofloxacin:
Information on overdosage with ofloxacin is limited. One incident of accidental overdosage has been reported. In this case, an adult female received 3 grams of ofloxacin intravenously over 45 minutes. A blood sample obtained 15 minutes after the completion of the infusion revealed an ofloxacin level of 39.3 mcg/mL. In 7 hours, the level had fallen to 16.2 mcg/mL, and by 24 hours to 2.7 mcg/mL. During the infusion, the patient developed drowsiness, nausea, dizziness, hot and cold flushes, subjective facial swelling and numbness, slurring of speech, and mild-to-moderate disorientation. All complaints except the dizziness subsided within 1 hour after discontinuation of the infusion. The dizziness, most bothersome while standing, resolved in approximately 9 hours. Laboratory testing reportedly revealed no clinically significant changes in routine parameters in this patient.
Ornidazole:
In cases of overdosage, the symptoms mentioned under Undesirable Effects occur in a more severe form. No specific antidote is known. The administration of diazepam is recommended if cramps occur. DOSAGE AND ADMINISTRATION:
5ml twice daily or as directed by doctor.
PACKAGING:Glass bottle of 100ml /carton box with measuring plastic cup.
STORAGE CONDIIONS: Store at room temperature(15-30)°C. RX Only.